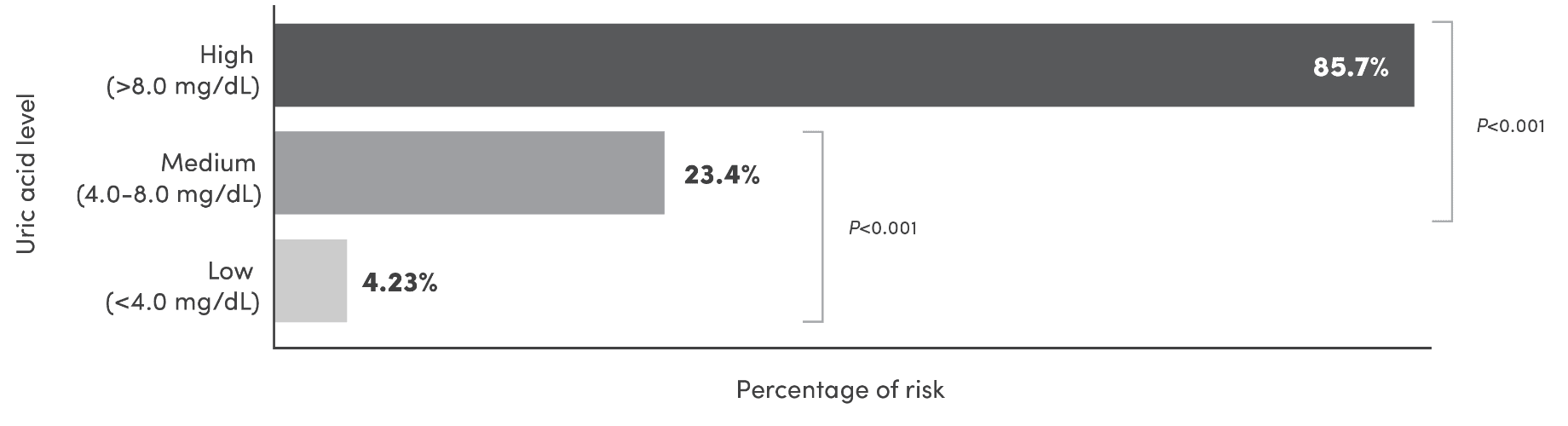
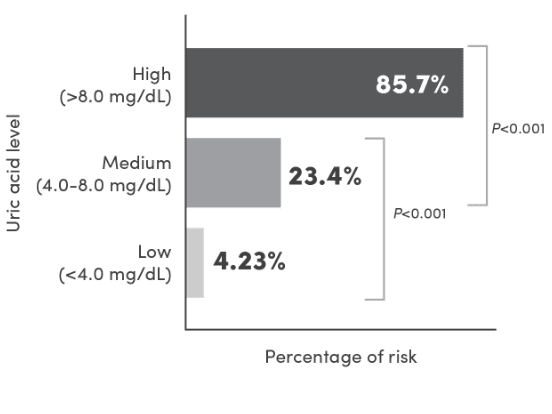
There are a wide range of patient and tumor-specific factors that can increase the risk of tumor lysis syndrome (TLS) and hyperuricemia in your patients1-3
![]()
High tumor burden
![]()
Elevated uric acid
levels at baseline
![]()
Bulky disease
![]()
Elevated WBC count
![]()
Lymph node
involvement
![]()
Bone marrow
involvement
![]()
Renal disease or renal
involvement by tumor
This is not a comprehensive list of all potential risk factors.
Are your patients at higher risk for TLS than you think?
Use this Risk Assessment Tool to help you identify patients who may be at risk for TLS
VISIT NOW
ALC=absolute lymphocyte count; CLL=chronic lymphocytic leukemia; CrCl=creatinine clearance; SLL=small lymphocytic lymphoma; WBC=white blood cell.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. V.3.2022. ©National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed August 26, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2. Wilson FP, Berns JS. Onco-nephrology: tumor lysis syndrome. Clin J Am Soc Nephrol. 2012;7(10):1730-1739. 3. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas. V.4.2022. ©National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed August 26, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 4. Cairo MS. Prevention and treatment of hyperuricemia in hematological malignancies. Clin Lymphoma. 2002;3(S1):S26-S31. 5. Edeani A, Shirali A. Chapter 4: Tumor Lysis Syndrome. Onco-Nephrology Curriculum. American Society of Nephrology. 2016. https://www.asnonline.org/education/distancelearning/curricula/onco/Chapter4.pdf. Accessed August 26, 2022. 6. Venclexta [prescribing information]. East Windsor, NJ: Acrotech Biopharma LLC; 2020. 7. Imbruvica [prescribing information]. Horsham, PA: Janssen Biotech, Inc.; 2020. 8. Yescarta [prescribing information]. Santa Monica, CA: Kite Pharma, Inc; 2022. 9. Revlimid [prescribing information]. Summit, NJ: Celgene Corporation; 2021. 10. Bendeka [prescribing information]. Parsippany, NJ: Teva Pharmaceuticals; 2021. 11. Gleevec [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2022. 12. Gazyva [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2022. 13. Thalomid [prescribing information]. Summit, NJ: Celgene Corporation; 2021. 14. Marqibo [prescribing information]. East Windsor, NJ: Acrotech Biopharma LLC; 2020. 15. Sprycel [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2021. 16. Pomalyst [prescribing information]. Summit, NJ: Celgene Corporation; 2021. 17. Doxorubicin hydrochloride [prescribing information]. New York, NY: Pfizer Labs; 2013. 18. Tasigna [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021. 19. Rituxan [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2021. 20. Adcetris [prescribing information]. Bothell, WA: Seagen Inc.; 2022. 21. Ninlaro [prescribing information]. Cambridge, MA: Millennium Pharmaceuticals, Inc.; 2021. 22. Tibsovo [prescribing information]. Boston, MA: Servier Pharmaceuticals LLC; 2021. 23. Kyprolis [prescribing information]. Thousand Oaks, CA: Onyx Pharmaceuticals, Inc.; 2021. 24. Velcade [prescribing information]. Lexington, MA: Takeda Pharmaceuticals America, Inc.; 2021. 25. Istodax [prescribing information]. Summit, NJ: Celgene Corporation; 2021. 26. Blincyto [prescribing information]. Thousand Oaks, CA: Amgen Inc.; 2022. 27. Polivy [prescribing information]. South San Francisco, CA: Genentech, Inc.; 2020. 28. Kymriah [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2022.