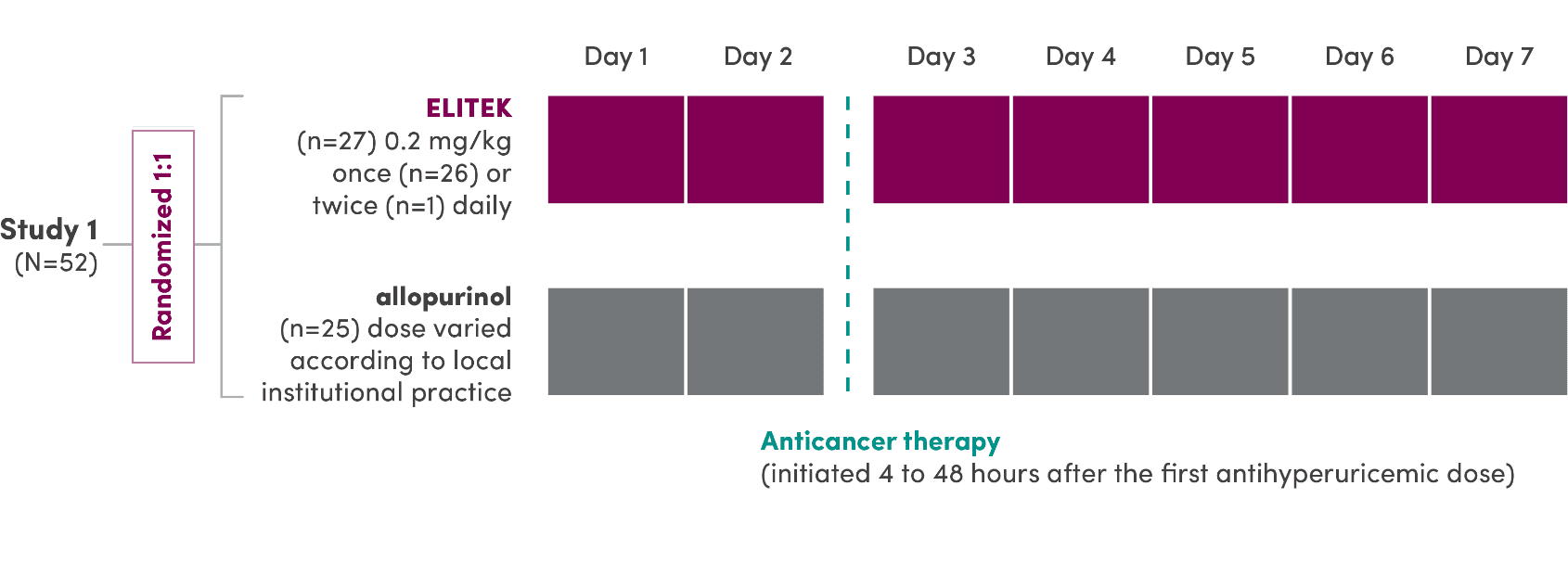
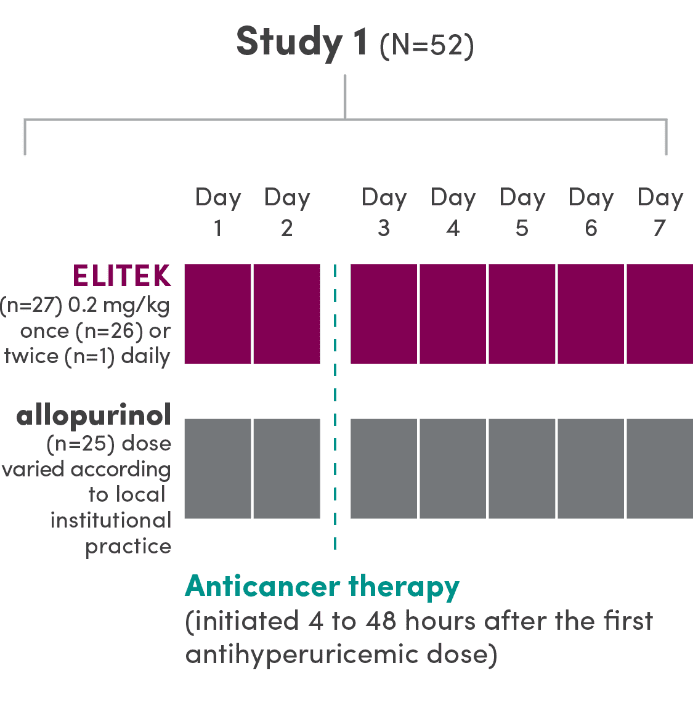
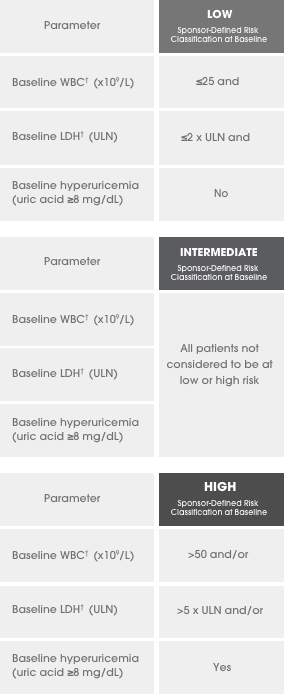
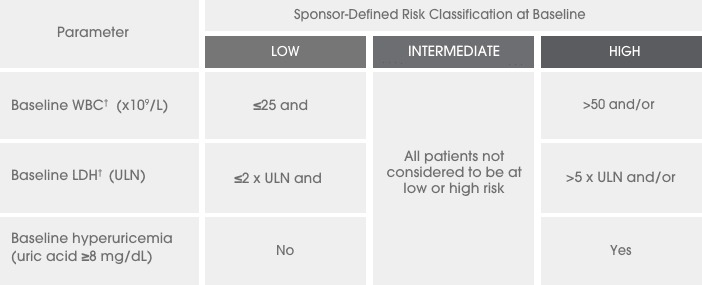
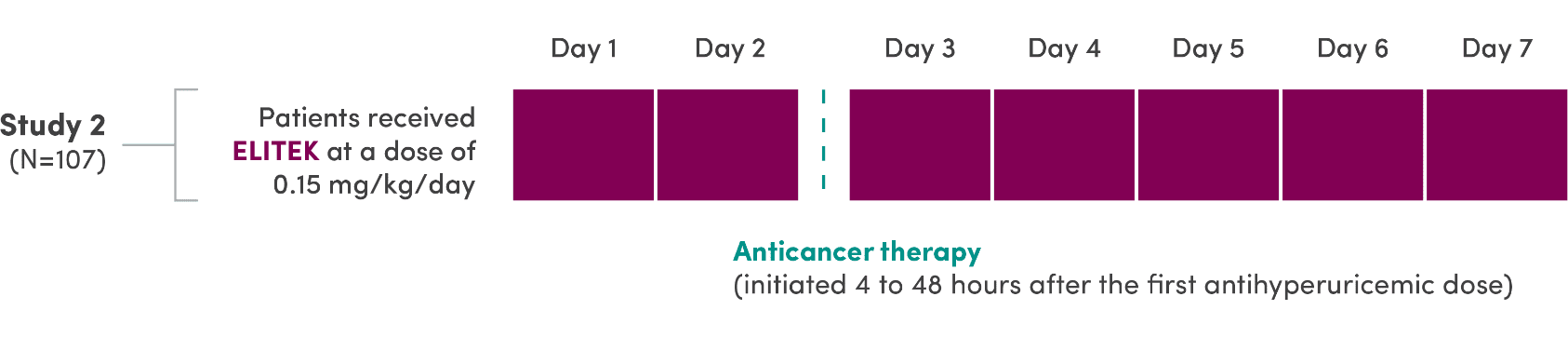
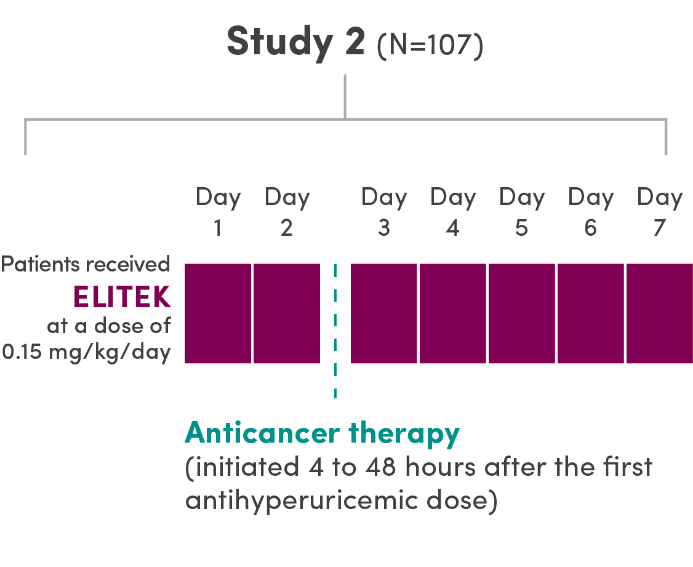
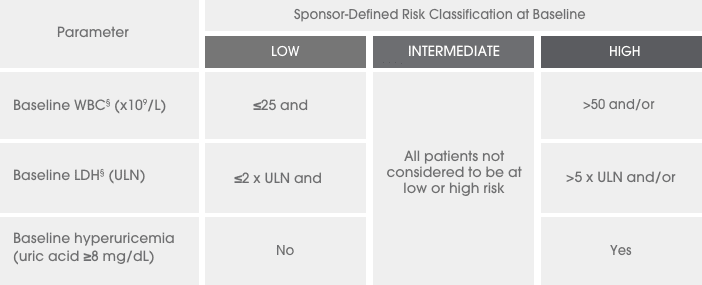
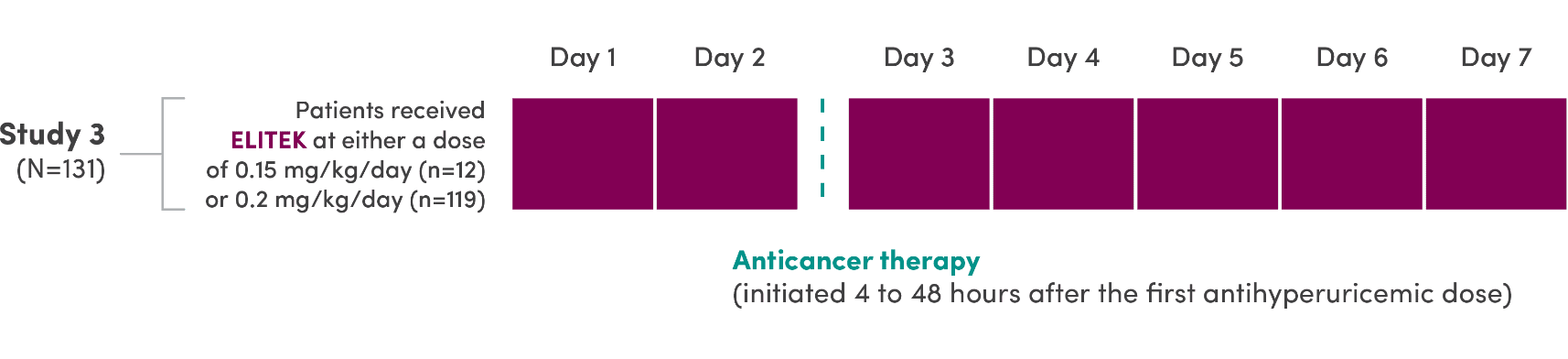
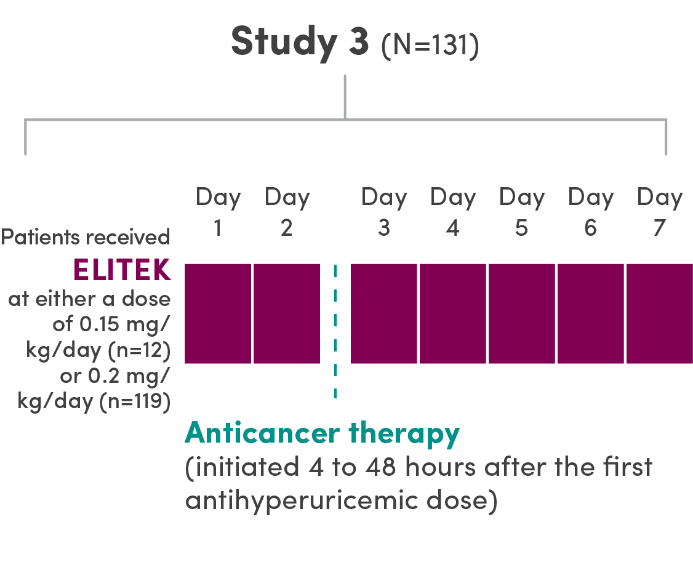
A series of 3 clinical trials was conducted
- ELITEK was administered to 265 patients (pooled) with hematologic malignancies1
- 93% (246/265) of patients (pooled) enrolled in these clinical trials were pediatric1
- 77% (200/261) of evaluable patients (pooled) had normal uric acid levels (<8 mg/dL) at baseline1
- ELITEK was administered prior to and concurrent with antitumor therapy1
- 95% (251/265) of patients (pooled) were administered a 30-minute infusion once daily; the others (14/265) received infusions twice daily1

EXPERT REVIEW VIDEO
Watch Dr. Jiajoyce R. Conway explain how pediatric patients with hematologic malignancies responded to treatment with ELITEK across 3 clinical trials
ELITEK Pediatric Brochure
Reference the Pediatric Brochure to see the ELITEK trial designs and results for a pediatric patient population
DOWNLOAD NOW
See how these pediatric patients responded to treatment with ELITEK
ALL=acute lymphocytic leukemia; ECOG PS=Eastern Cooperative Oncology Group Performance Scale; FAB=French-American-British; IV=intravenous; LDH=lactate dehydrogenase; NHL=non-Hodgkin’s lymphoma; ULN=upper limit of normal; WBC=white blood cell.
References: 1. ELITEK [prescribing information]. Bridgewater, NJ: sanofi-aventis U.S. LLC. 2. Goldman SC, Holcenberg JS, Finkelstein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998-3003. 3. Data on file. Bridgewater, NJ: sanofi-aventis U.S. LLC.